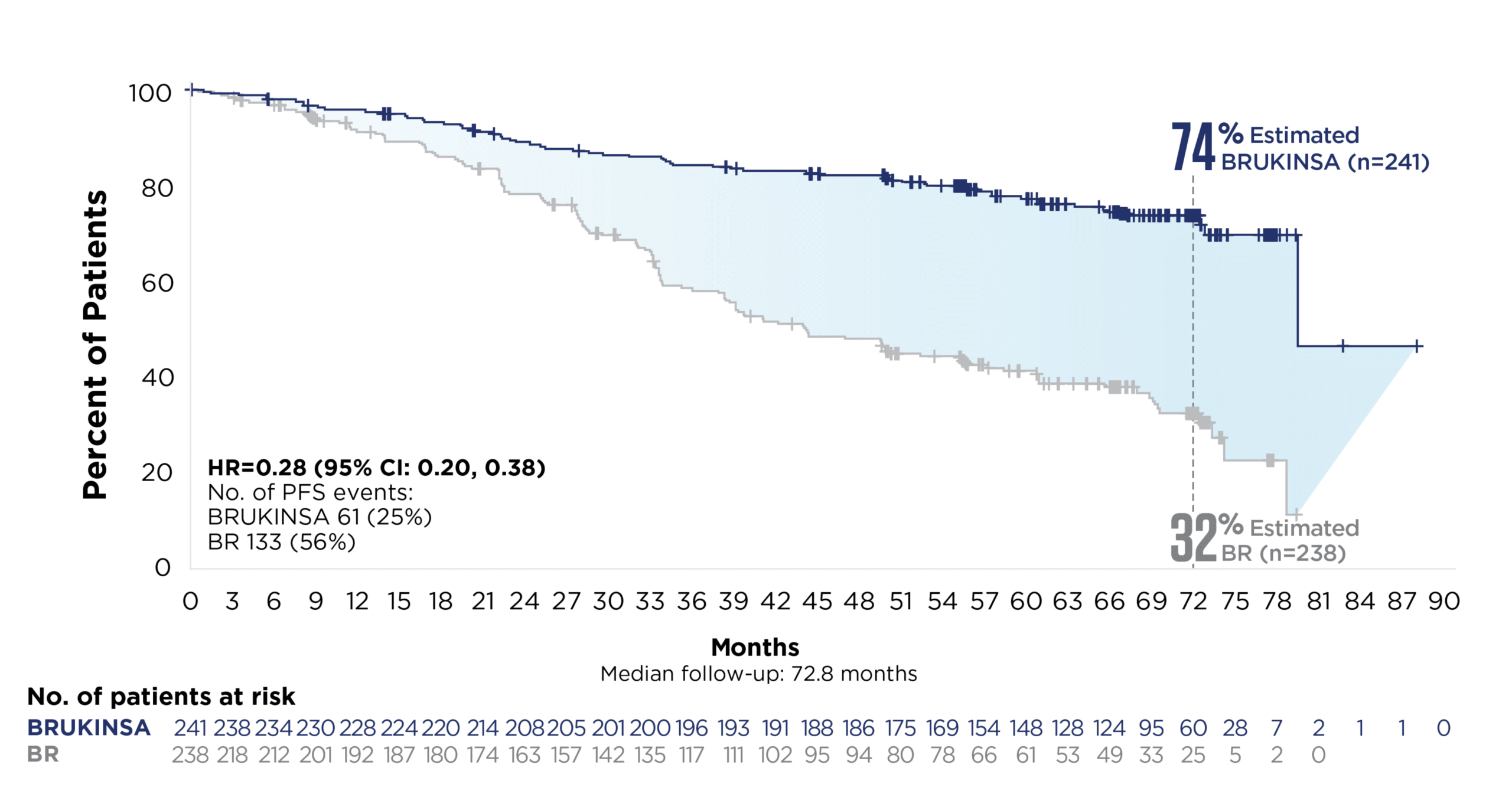
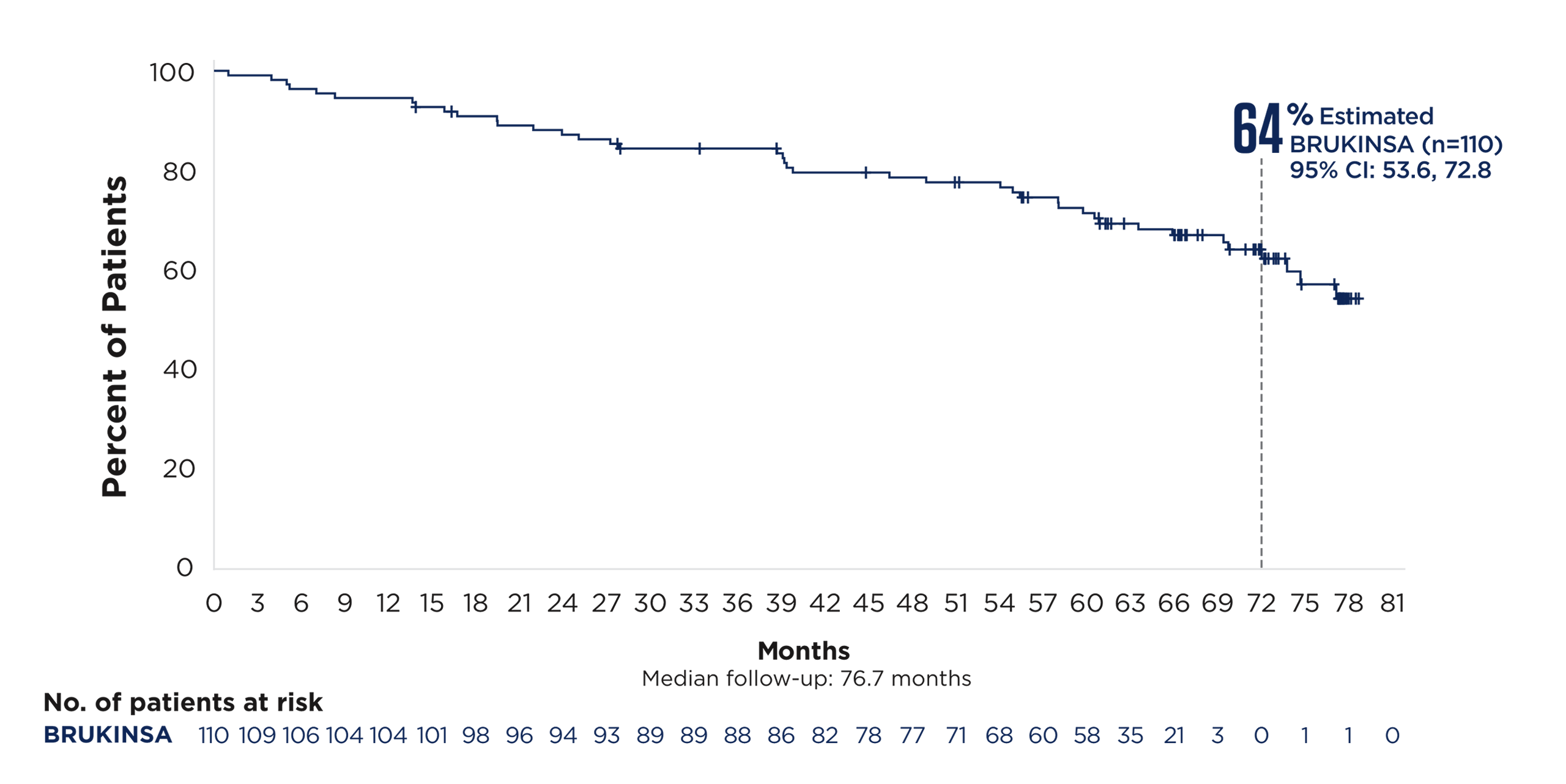
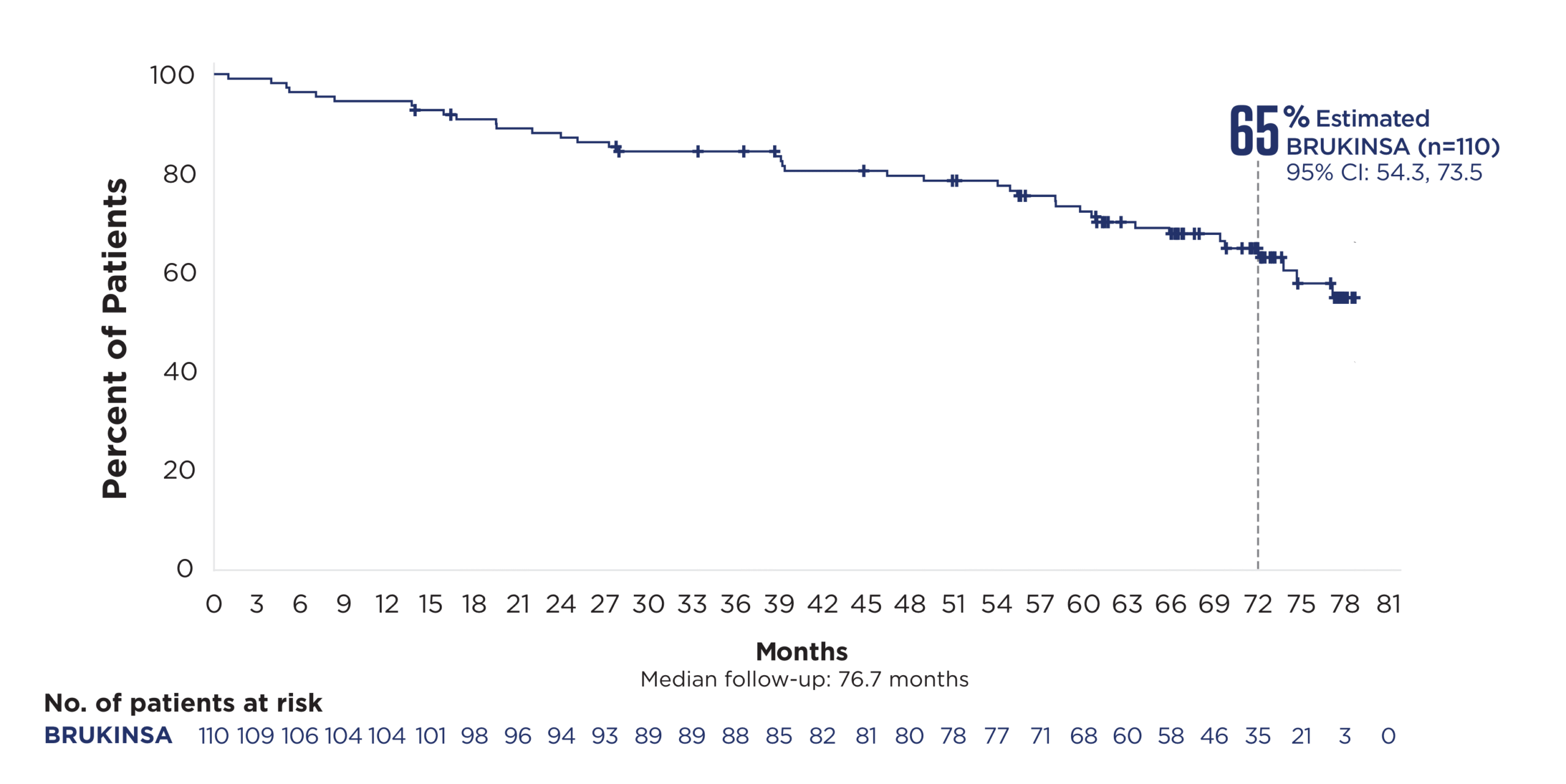
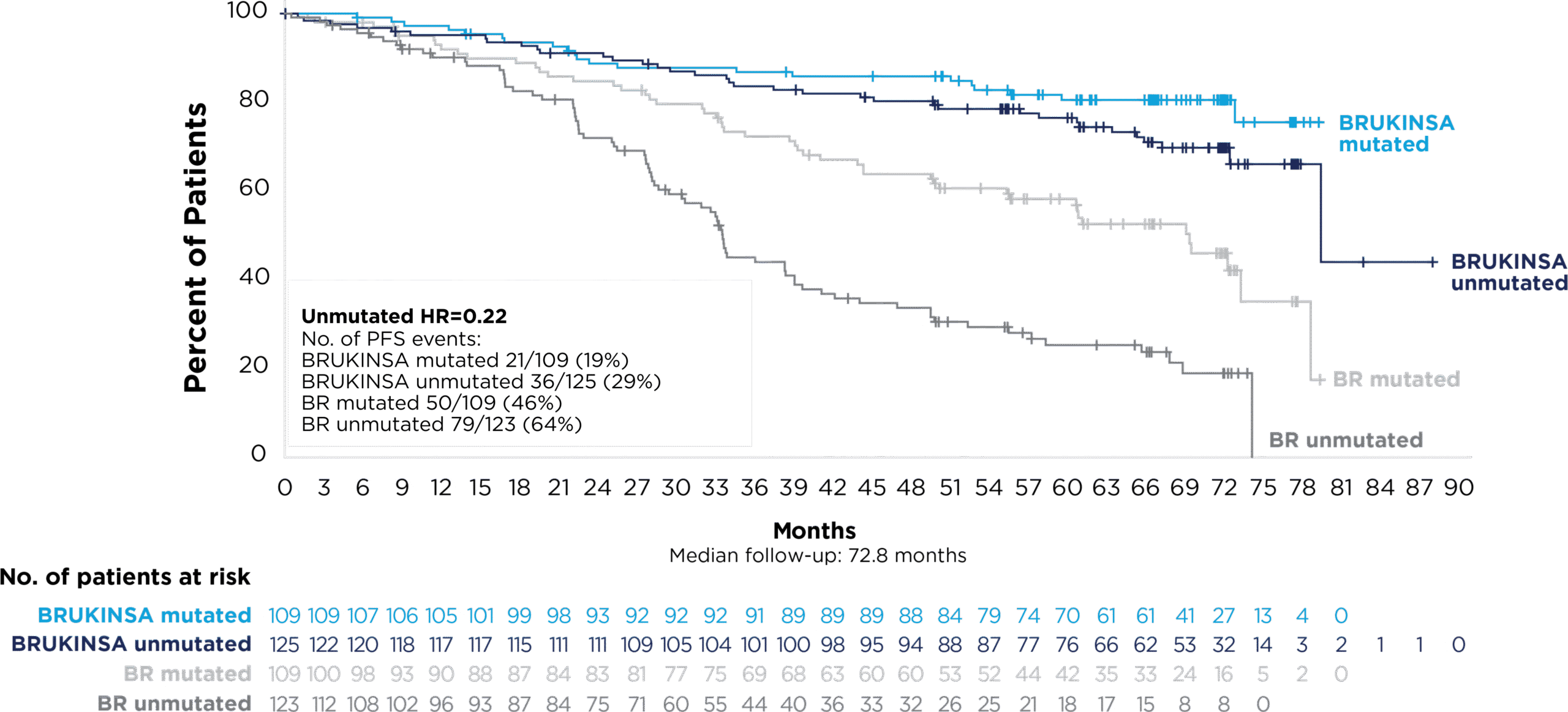
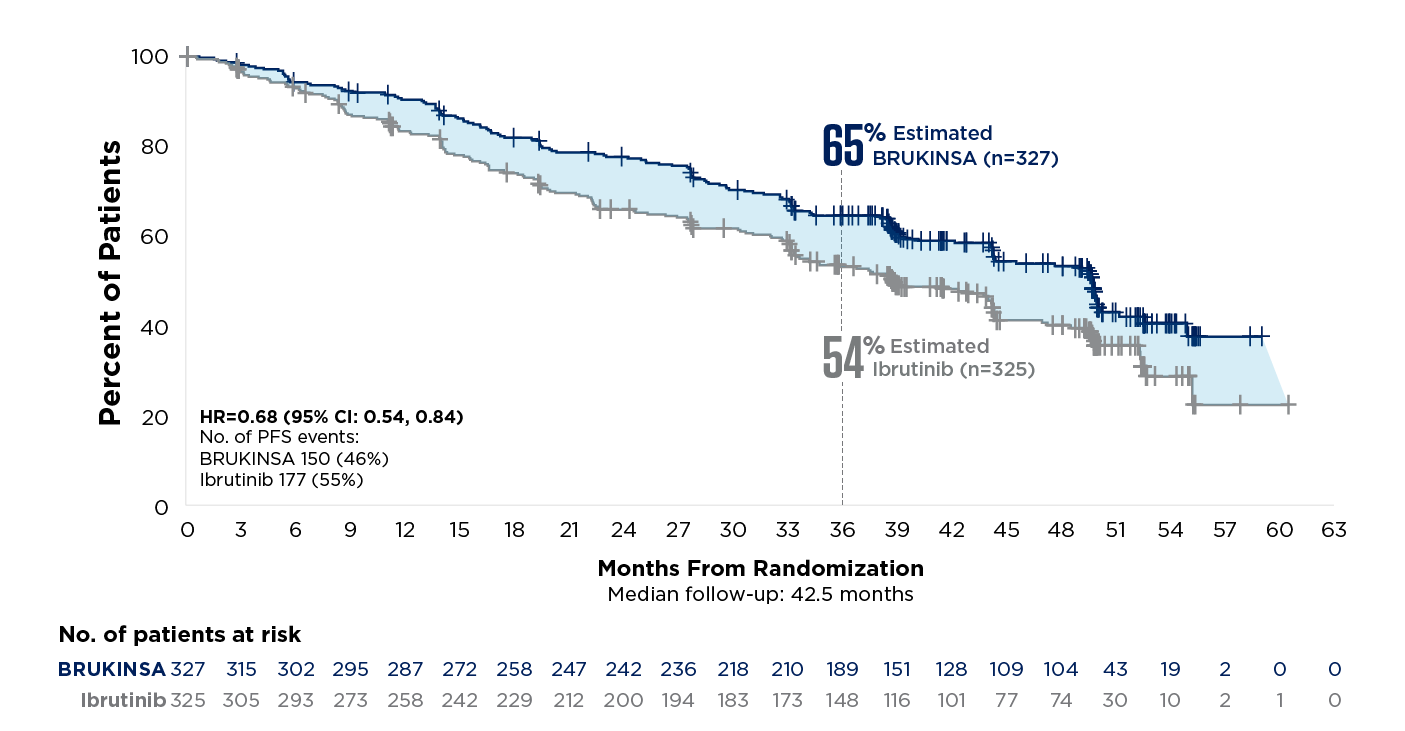
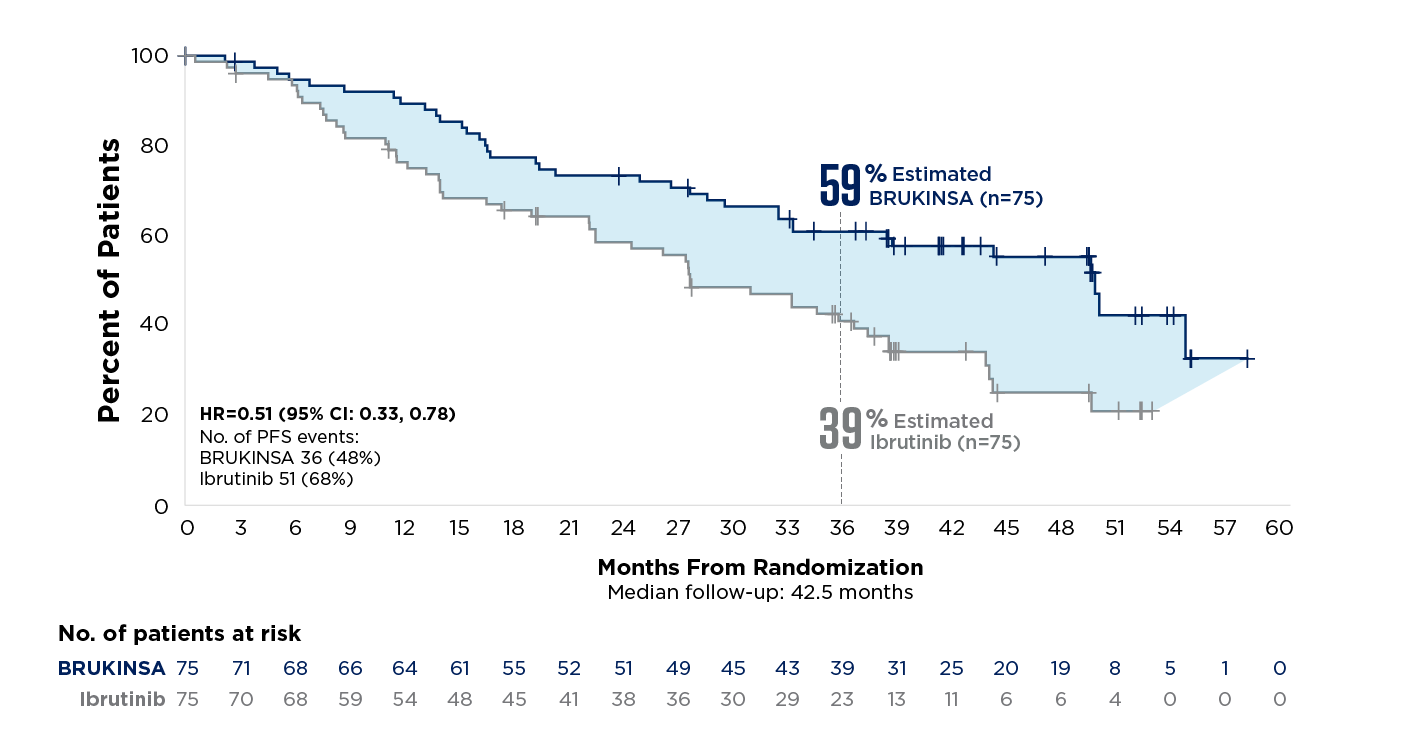
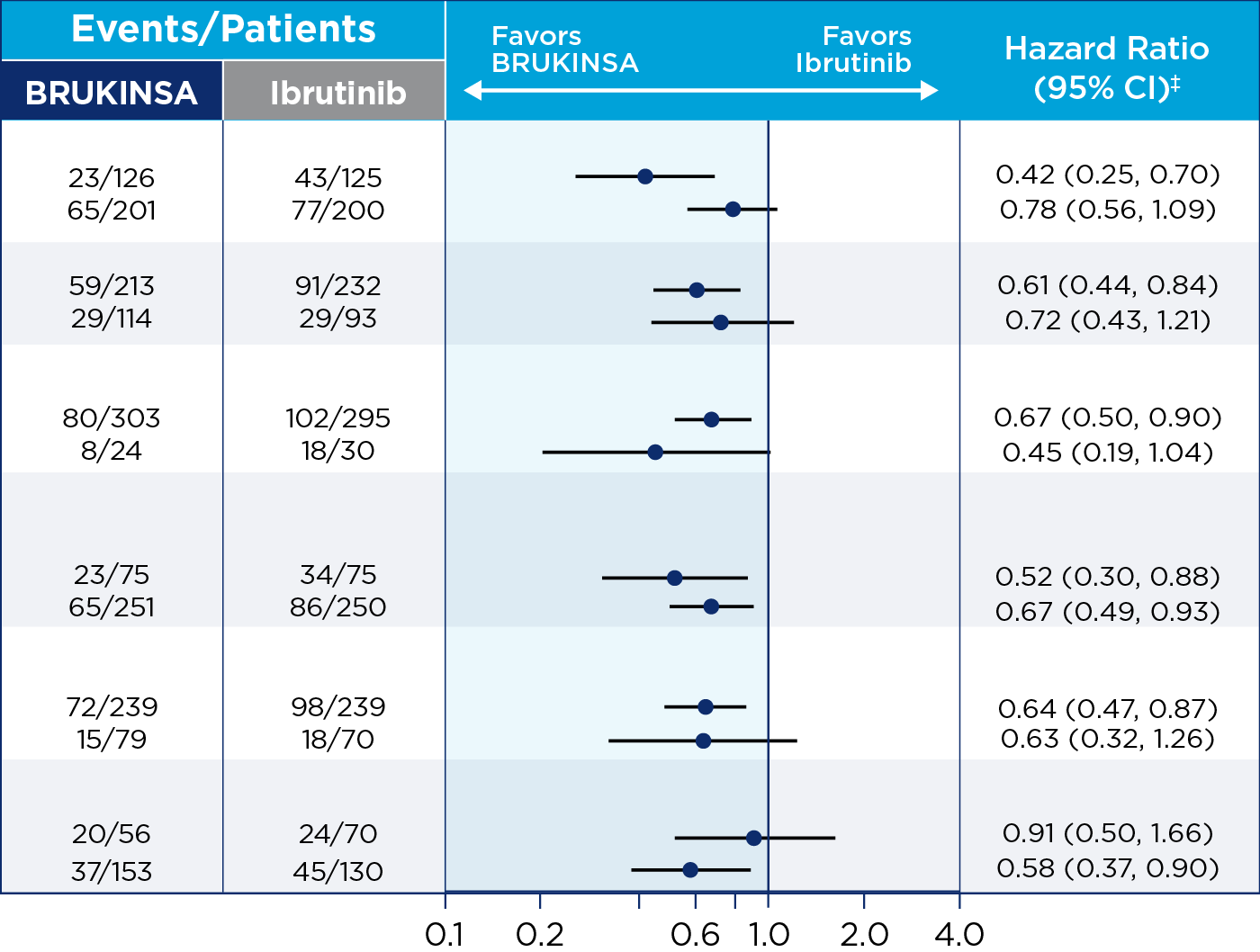
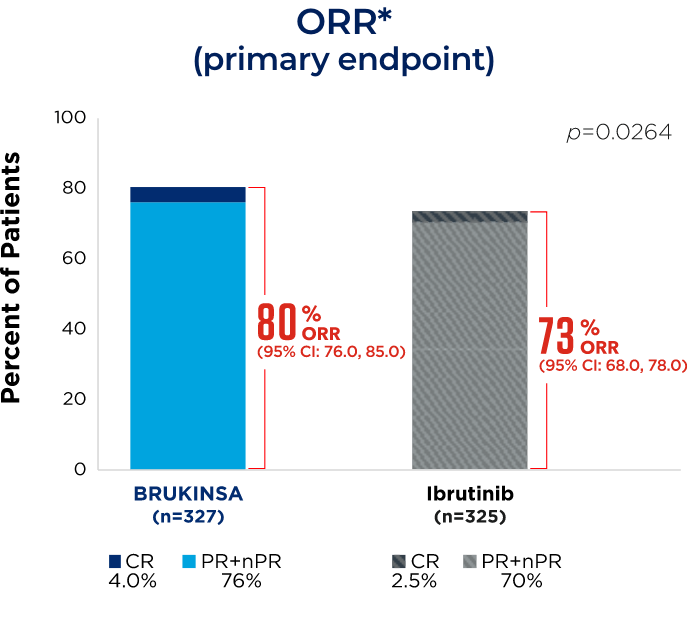
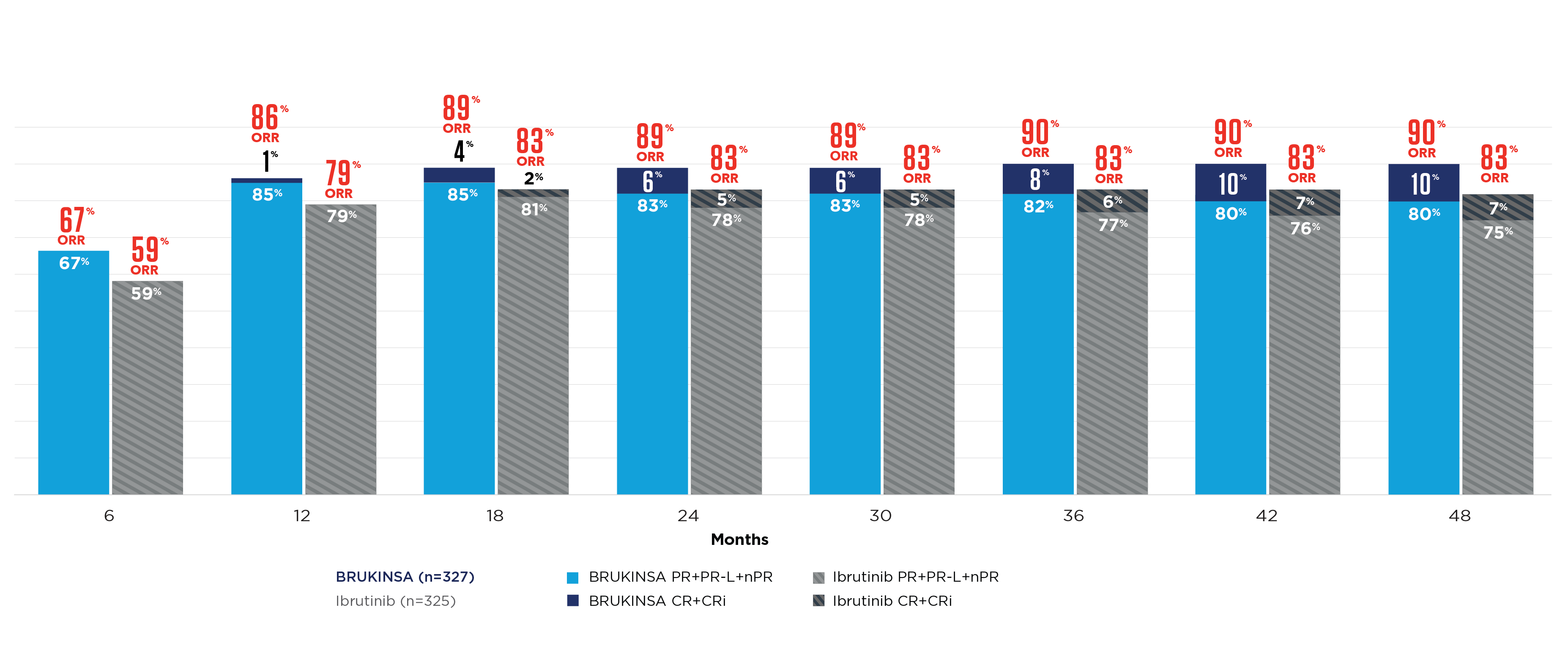
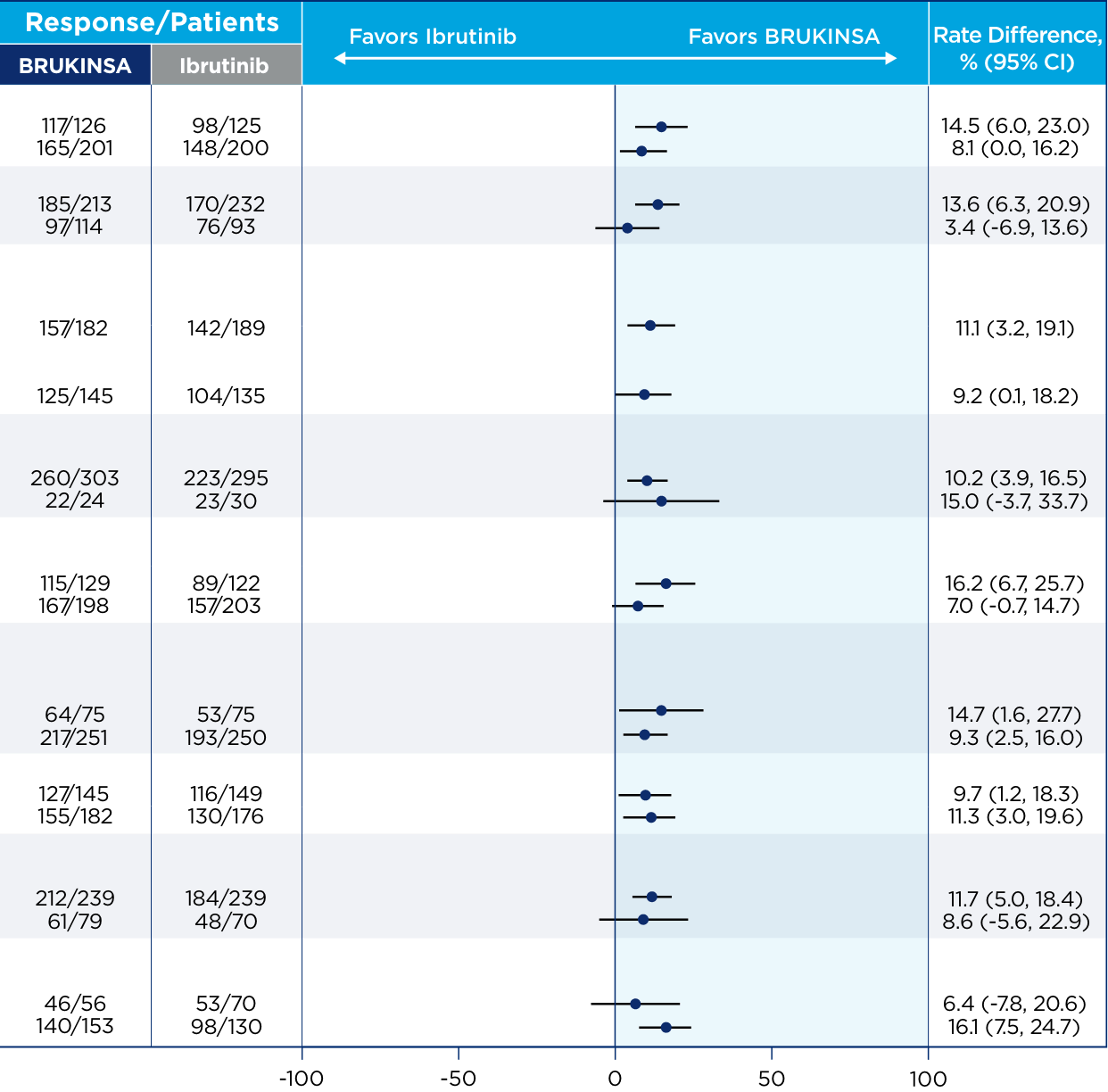
References: 1. BRUKINSA. Package insert. BeOne Medicines USA, Inc.; 2025. 2. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031-1043. 3. Tam CS, Munir T, Robak T, et al. Sustained efficacy of zanubrutinib vs bendamustine + rituximab in treatment-naive chronic lymphocytic leukemia/small lymphocytic lymphoma and continued favorable survival in non-randomized patients with del(17p): 6-year follow-up in the phase 3 SEQUOIA study. Presented at: American Society of Hematology (ASH) Annual Meeting and Exposition; December 6-9, 2025. Poster 2129. 4. Shadman M, Munir T, Ma S, et al. Combination of zanubrutinib + venetoclax for treatment-naive CLL/SLL: results in SEQUOIA Arm D. Presented at: American Society of Clinical Oncology (ASCO) 2025 Annual Meeting; May 30-June 3, 2025. 5. Davids MS, Ryan CE, Lampson BL, et al. Phase II study of acalabrutinib, venetoclax, and obinutuzumab in a treatment-naïve chronic lymphocytic leukemia population enriched for high-risk disease. J Clin Oncol. 2025;43(7):788-799. 6. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib-obinutuzumab improves survival vs chemoimmunotherapy in treatment-naive CLL in the 6-year follow-up of ELEVATE-TN. Blood. 2025;146(11):1276-1285. 7. Al-Sawaf O, Robrecht S, Zhang C, et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized phase 3 CLL14 study. Blood. 2024;144(18):1924-1935. 8. Wierda WG, Jacobs R, Barr PM, et al. Outcomes in high-risk subgroups after fixed-duration ibrutinib + venetoclax for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): up to 5.5 years of follow-up in the phase 2 CAPTIVATE study. J Clin Oncol. 2024;42(16[suppl]). Abstract 7009. 9. Shadman M, Munir T, Robak T, et al. Zanubrutinib versus bendamustine and rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma: median 5-year follow-up of SEQUOIA. J Clin Oncol. 2025;43(7):780-787. 10. Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319-332. 11. Brown JR, Eichhorst B, Lamanna N, et al. Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: final comparative analysis of ALPINE. Blood. 2024;144(26):2706-2717. 12. Data on file. BeiGene USA, Inc.