IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Hemorrhage
Fatal and serious hemorrhage has occurred in patients with hematological malignancies treated with BRUKINSA. Grade 3 or higher hemorrhage including intracranial and
gastrointestinal hemorrhage, hematuria, and hemothorax was reported in 3.8% of patients treated with BRUKINSA in clinical trials, with fatalities occurring in 0.2% of
patients. Bleeding of any grade, excluding purpura and petechiae, occurred in 32% of patients.
Bleeding has occurred in patients with and without concomitant antiplatelet or anticoagulation therapy. Coadministration of BRUKINSA with antiplatelet or anticoagulant
medications may further increase the risk of hemorrhage.
Monitor for signs and symptoms of bleeding. Discontinue BRUKINSA if intracranial hemorrhage of any grade occurs. Consider the benefit-risk of withholding BRUKINSA for
3-7 days before and after surgery depending upon the type of surgery and the risk of bleeding.
Infections
Fatal and serious infections (including bacterial, viral, or fungal infections) and opportunistic infections have occurred in patients with hematological malignancies
treated with BRUKINSA. Grade 3 or higher infections occurred in 26% of patients, most commonly pneumonia (7.9%), with fatal infections occurring in 3.2% of patients.
Infections due to hepatitis B virus (HBV) reactivation have occurred.
Consider prophylaxis for herpes simplex virus, pneumocystis jirovecii pneumonia, and other infections according to standard of care in patients who are at increased
risk for infections. Monitor and evaluate patients for fever or other signs and symptoms of infection and treat appropriately.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (21%), thrombocytopenia (8%) and anemia (8%) based on laboratory measurements, developed in patients treated with
BRUKINSA. Grade 4 neutropenia occurred in 10% of patients, and Grade 4 thrombocytopenia occurred in 2.5% of patients.
Monitor complete blood counts regularly during treatment and interrupt treatment, reduce the dose, or discontinue treatment as warranted. Treat using growth factor or
transfusions, as needed.
Second Primary Malignancies
Second primary malignancies, including non-skin carcinoma, have occurred in 14% of patients treated with BRUKINSA. The most frequent second primary malignancy was
non-melanoma skin cancers (8%), followed by other solid tumors in 7% of the patients (including melanoma in 1% of patients) and hematologic malignancies (0.7%). Advise
patients to use sun protection and monitor patients for the development of second primary malignancies.
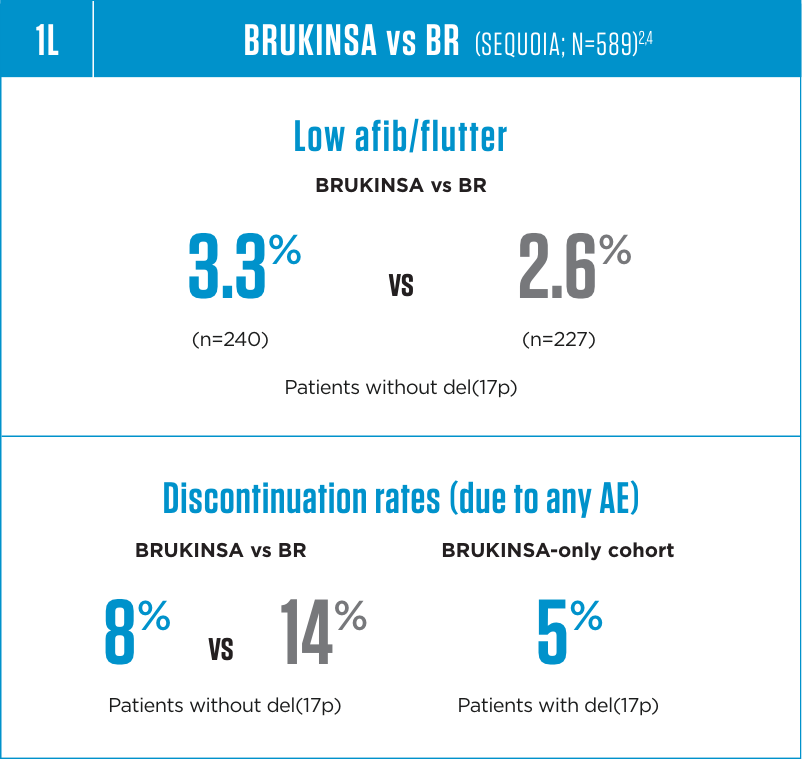
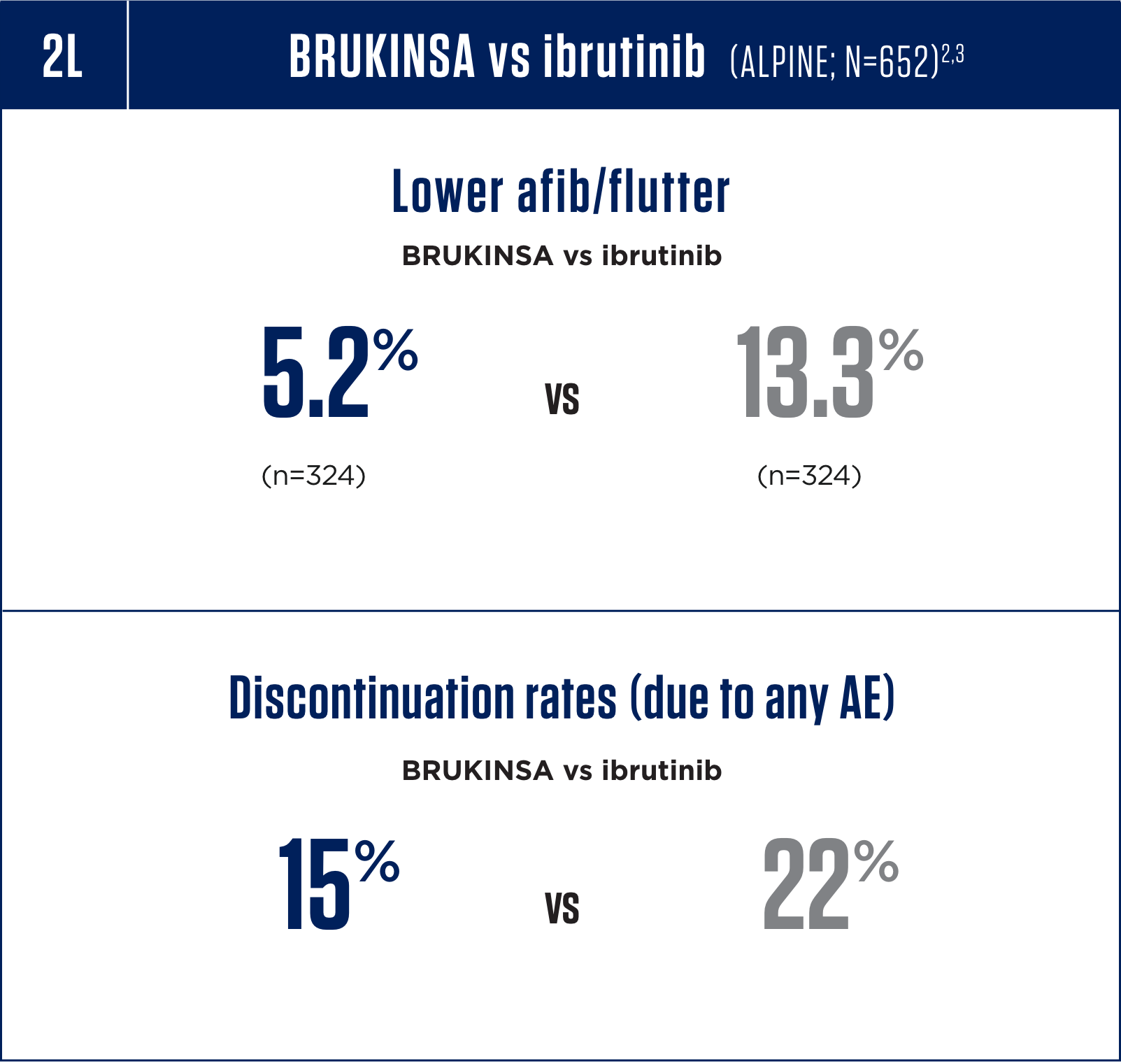
Cardiac Arrhythmias
Serious cardiac arrhythmias have occurred in patients treated with BRUKINSA. Atrial fibrillation and atrial flutter were reported in 4.4% of patients treated with
BRUKINSA, including Grade 3 or higher cases in 1.9% of patients. Patients with cardiac risk factors, hypertension, and acute infections may be at increased risk. Grade
3 or higher ventricular arrhythmias were reported in 0.3% of patients.
Monitor for signs and symptoms of cardiac arrhythmias (e.g., palpitations, dizziness, syncope, dyspnea, chest discomfort), manage appropriately, and consider the risks
and benefits of continued BRUKINSA treatment.
Hepatotoxicity, Including Drug-Induced Liver Injury
Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of drug-induced liver injury (DILI), has occurred in patients treated with Bruton
tyrosine kinase inhibitors, including BRUKINSA.
Evaluate bilirubin and transaminases at baseline and throughout treatment with BRUKINSA. For patients who develop abnormal liver tests after BRUKINSA, monitor more
frequently for liver test abnormalities and clinical signs and symptoms of hepatic toxicity. If DILI is suspected, withhold BRUKINSA. Upon confirmation of DILI,
discontinue BRUKINSA.
Embryo-Fetal Toxicity
Based on findings in animals, BRUKINSA can cause fetal harm when administered to a pregnant woman. Administration of zanubrutinib to pregnant rats during the period of
organogenesis caused embryo-fetal toxicity, including malformations at exposures that were 5 times higher than those reported in patients at the recommended dose of 160
mg twice daily. Advise women to avoid becoming pregnant while taking BRUKINSA and for 1 week after the last dose. Advise men to avoid fathering a child during treatment
and for 1 week after the last dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of
the potential hazard to a fetus.
ADVERSE REACTIONS
The most common adverse reactions (≥30%), including laboratory abnormalities, in patients who received BRUKINSA (N=1729) are decreased neutrophil count (51%), decreased
platelet count (41%), upper respiratory tract infection (38%), hemorrhage (32%), and musculoskeletal pain (31%).
DRUG INTERACTIONS
CYP3A Inhibitors: When BRUKINSA is coadministered with a strong CYP3A inhibitor, reduce BRUKINSA dose to 80 mg once daily. For coadministration with a
moderate CYP3A inhibitor, reduce BRUKINSA dose to 80 mg twice daily.
CYP3A Inducers: Avoid coadministration with strong or moderate CYP3A inducers. Dose adjustment may be recommended with moderate CYP3A inducers.
SPECIFIC POPULATIONS
Hepatic Impairment: The recommended dose of BRUKINSA for patients with severe hepatic impairment is 80 mg orally twice daily.